Teeth can glow or fluoresce under ultraviolet light due to interactions between UV photons and minerals in tooth enamel and dentin. This fluorescence has intrigued many people who shine a UV or blacklight on their smile. What causes this phenomenon? While simplified explanations invoke the enamel’s makeup, the actual reasons involve complex photonic and crystalline physics.
Mechanisms Behind Dental Fluorescence
In-depth research has revealed various processes responsible for making teeth glow:
1. Ultraviolet Light Effects
When UV photons enter enamel, they excite electrons within the hydroxyapatite crystals of the enamel matrix. Excited electrons jump to higher energy orbits then relax back to ground state. This relaxation releases energy as visible light photons, causing fluorescence.
Shortwave UVB and UVC radiation provides the highest energy photons that produce the brightest fluorescence. However, UVA near the 365-370 nm range elicits optimal tooth fluorescence with lower risk of damage from higher energy UV.
2. Crystal Structure
Hydroxyapatite crystals contain phosphates, hydroxides, and calcium ions arranged in a hexagonal structure. This unique configuration of components exhibits more intense fluorescence compared to other biological minerals.
The crystals act as hosts for impurities like carbonates that occupy substitutional sites. The impurity ions enhance phosphor effects to increase fluorescence.
3. Enamel Permeability
Whitening agents and natural enamel breakdown increase porosity and permeability. This allows deeper penetration of UV light into enamel to generate more uniform and intense fluorescence.
Without permeability, most fluorescence arises only near the outer enamel surface. Increasing porosity fluorescent emissions emerge from the bulk enamel interior.
4. Quantum Processes
Beyond simple electron excitation, quantum effects within the hydroxyapatite matrix also enable fluorescence. Proton transfer and charge recombination produce alternate relaxation pathways upon UV photon absorption.
Additionally, quantum coupling between adjacent hydroxyapatite crystals generates new hybrid states that fluoresce at higher wavelengths than the crystals alone.
Factors That Influence Fluorescence
Multiple variables affect how brightly teeth will fluoresce:
1. Age
Enamel wears down and thins over time, decreasing its capacity to absorb and transmit UV light. Older teeth exhibit lower intensity fluorescence.
2. Staining
Chromophores from stains occupy spaces between crystals. This blocks UV light penetration and fluorescence unless stains are removed by cleaning.
3. Trauma
Cracks and fractures disrupt the enamel crystalline structure. With less organized matrix, fewer emissions occur uniformly across damaged areas.
4. Chemical Exposure
Chemicals like tetracyclines displace mineral components in enamel. Replaced hydroxyapatite cannot produce fluorescence, so brightness diminishes.
5. Whitening Treatments
Bleaching opens pores in enamel crystals to allow deeper UV light penetration. This enhances fluorescence, but effects diminish as pores reclose over weeks.
6. Radiation Exposure
Ionizing radiation disrupts crystals and the matrix structure. With less uniform enamel architecture, fluorescence decreases.
7. Dental Work
Materials like composites and porcelain do not fluoresce. But surrounding enamel interface and some cements still exhibit fluorescence.
8. Systemic Factors
Diseases that disrupt enamel mineralization and maturation during development can reduce maximal fluorescence capacity.
Applications of Tooth Fluorescence
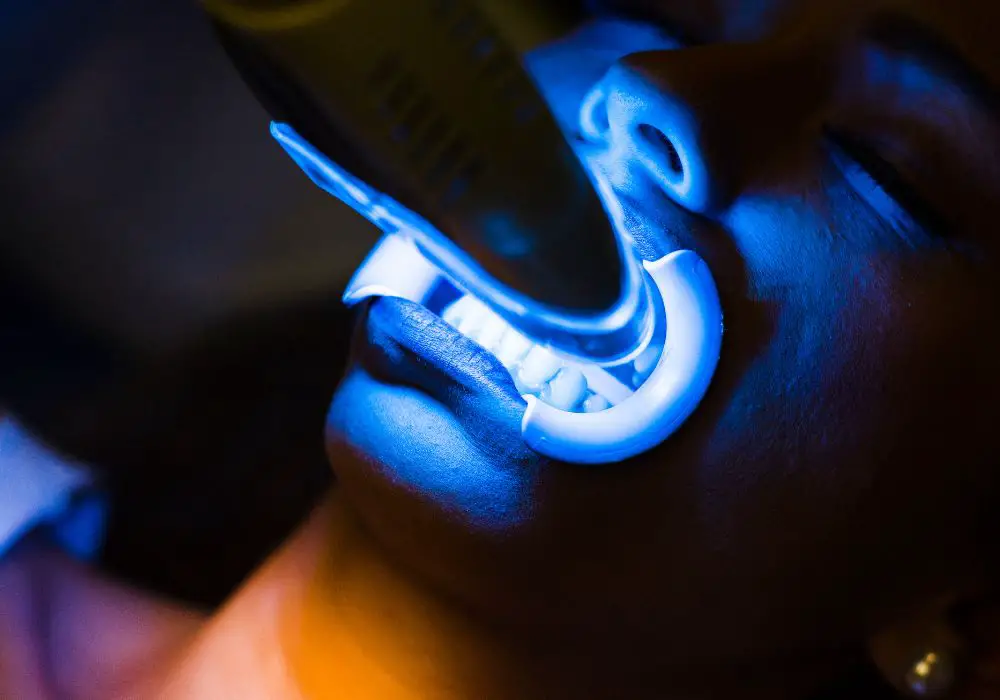
Beyond aesthetic and entertainment novelty, dental fluorescence has real clinical applications:
1. Detection of Dental Caries
Degraded, porous enamel fluoresces less than healthy enamel. UV inspection highlights developing lesions for early intervention.
2. Analysis of Restorations
Non-fluorescent composites and porcelains contrast against fluorescing natural enamel. This reveals microleakage or recurrent decay at restorative margins.
3. Assessment of Hygiene
Plaque and calculus obscure natural fluorescence until removed by cleaning. Return of brightness indicates hygiene efficacy.
4. Forensic Identification
Matching patterns of fluorescent spots, fillings, and dental work helps identify unknown remains by comparing antemortem and postmortem records.
5. Monitoring of Growth and Development
In children and adolescents, UV inspection tracks progressive enamel thickness and maturation based on increasing fluorescence.
6. Age Estimation
Measuring fluorescence intensity decline in adults can indicate relative age due to enamel wear over time.
7. Researching Mineralization
Studying how fluorescence changes with nutritional or environmental variation provides insight on enamel mineralization.
Misconceptions About Tooth Fluorescence
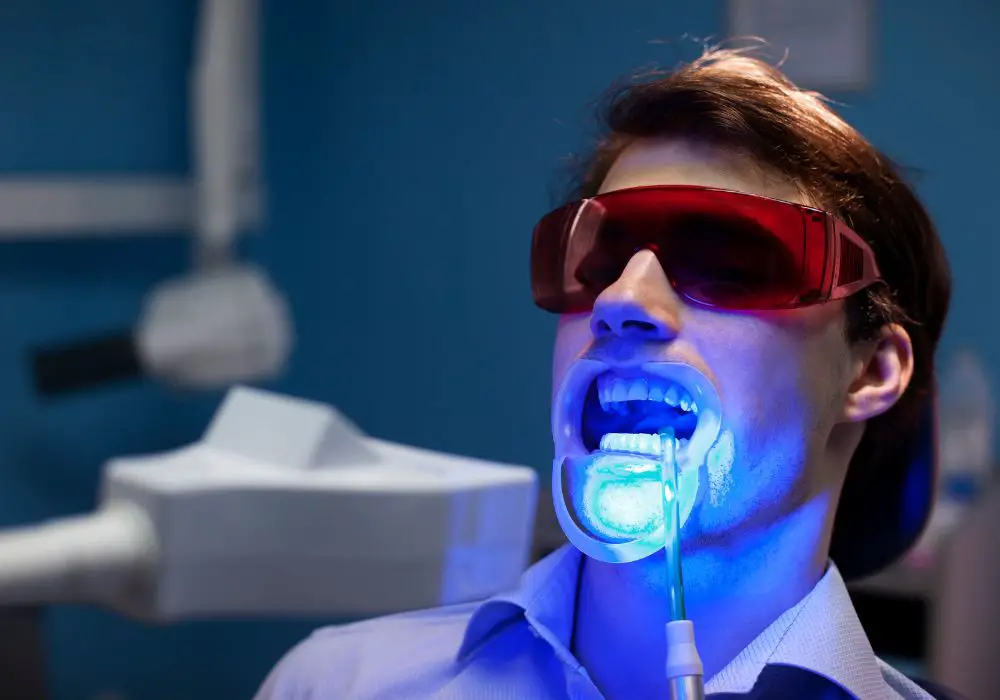
Myth: All healthy teeth fluoresce strongly.
Fact: Natural fluorescence varies widely depending on enamel thickness, mineralization levels, and other factors. Weak fluorescence does not indicate disease.
Myth: UV exposure makes teeth fluorescent.
Fact: UV light simply elicits innate fluorescence from enamel crystals. UV does not produce the capacity to fluoresce and excessive exposure can damage teeth.
Myth: Old teeth fluoresce more than younger teeth.
Fact: Fluorescence decreases in older teeth as enamel wears down and thins with age.
Myth: Bleaching permanently increases fluorescence.
Fact: Whitening effects are temporary with greatest brightness in the first few days before tapering off over weeks.
Myth: Fluorescence only comes from enamel.
Fact: Dentin also produces fluorescence but less than enamel due to lower mineral content beneath the enamel layer.
Conclusion
Dental fluorescence originates from complex photonic, quantum, and crystalline effects when UV light interacts with hydroxyapatite and other minerals. Fluorescence varies naturally and changes with age and dental health factors. While a source of entertainment, fluorescence also serves clinical utility for health screening and treatment. With science-based insights, we can fully appreciate the marvels of a glowing smile.
Frequently Asked Questions
Why do some teeth fluoresce more than others?
The brightness of tooth fluorescence depends on variables like enamel thickness, porosity, mineral content, stain accumulation, and age. Teeth with thicker, more highly mineralized enamel and less staining exhibit the brightest fluorescence.
Do whitening products permanently increase fluorescence?
The effects of whitening treatments are temporary. Fluorescence peaks 1-2 days after bleaching, then decreases over subsequent weeks as enamel porosity declines back to baseline.
How does fluorescence help detect dental problems?
Fluorescence deficiencies highlight issues like demineralization, fracture lines, leaky restorations, plaque accumulation, and erosion to enable early diagnosis and intervention.
Does tooth fluorescence correlate with oral hygiene?
Yes, plaque and calculus block fluorescence until removed by brushing and flossing. Return of brightness indicates cleaner teeth. Stains from poor hygiene also reduce fluorescence.
What causes fluorescence to decrease with age?
As enamel wears down over time, it becomes thinner. This reduces its UV light absorbing capacity. Older teeth also accumulate more stains which impair fluorescence.